Performance Orthopedics of the Palm Beaches to Host Clinical Trial of FDA Approved Personalized Knee Implant
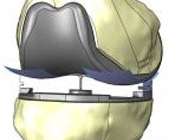
April 26, 2011 | PALM BEACH, Fla.–(EON: Enhanced Online News)–Dr. Gregory Martin of Performance Orthopedics of the Palm Beaches is participating in a ten year follow-up trial of the ConforMIS iUni® G2 knee resurfacing device, an FDA cleared implant for patients with osteoarthritic damage in a single compartment of the knee. Unlike traditional total knee replacement which replaces the entire joint, the ConforMIS partial knee resurfacing device allows for the targeted and minimally invasive treatment of just the diseased area of the knee in properly indicated patients.
“We are eager to take part in the iUni trial, which is designed to evaluate the long-term performance of this customized approach to unicompartmental knee replacement in treating osteoarthritis,” said Dr. Gregory Martin, Principal Investigator of the clinical trial at Performance Orthopedics of the Palm Beaches. “This clinical trial will be an important step to showcase the long-term impact that this personalized approach will deliver to our patients.”
Read the Full Article from Enhanced Online News